This chapter explores the physical properties of matter, its particulate nature, the three states of matter (solid, liquid, gas), and changes between states. You will understand characteristics of matter particles, diffusion, melting, boiling, evaporation, sublimation, and latent heat concepts that explain everyday phenomena.
Introduction
Everything in this universe is made up of material which scientists have named “matter”. The air we breathe, the food we eat, stones, clouds, stars, plants and animals, even a small drop of water or a particle of sand — everything is matter. All things occupy space and have mass. In other words, they have both mass and volume.
Historical Classification
Early Indian Philosophers:
- Classified matter in the form of five basic elements — the “Panch Tatva”:
- Air
- Earth
- Fire
- Sky
- Water
- According to them, everything living or non-living was made up of these five basic elements.
Ancient Greek Philosophers:
- Arrived at a similar classification of matter.
Modern Scientists:
- Have evolved two types of classification based on:
- Physical properties
- Chemical nature
- This chapter focuses on matter based on its physical properties.
- Chemical aspects will be covered in subsequent chapters.
Units of Measurement
- SI unit of mass: kilogram (kg)
- SI unit of volume: cubic metre (m³)
- Common unit of volume: litre (L)
- 1 L = 1 dm³
- 1 L = 1000 mL
- 1 mL = 1 cm³
1.1 Physical Nature of Matter
1.1.1 MATTER IS MADE UP OF PARTICLES
Historical Debate:
For a long time, two schools of thought prevailed regarding the nature of matter:
- One school believed matter to be continuous like a block of wood.
- The other thought that matter was made up of particles like sand.
Understanding Through Observation:
When we dissolve salt or sugar in water:
- The salt disappears from view.
- The water level does not change significantly.
- This happens because salt particles spread throughout water by occupying the spaces between water particles.
Conclusion:
- Matter is made up of tiny particles.
- These particles have spaces between them.
- When we dissolve salt in water, the particles of salt get into the spaces between particles of water.
1.1.2 HOW SMALL ARE THESE PARTICLES OF MATTER?
Experiment with Potassium Permanganate:
When 2-3 crystals of potassium permanganate are dissolved in 100 mL of water and then diluted repeatedly (5 to 8 times by taking 10 mL solution and adding it to 90 mL clear water each time):
- The water still remains coloured even after multiple dilutions.
- Just a few crystals can colour about 1000 L of water.
Alternative Experiment:
- Using 2 mL of Dettol instead of potassium permanganate.
- The smell can be detected even on repeated dilution.
Conclusion:
- There must be millions of tiny particles in just one crystal of potassium permanganate.
- These particles keep dividing into smaller and smaller particles.
- The particles of matter are very small — they are small beyond our imagination!
1.2 Characteristics of Particles of Matter
1.2.1 PARTICLES OF MATTER HAVE SPACE BETWEEN THEM
Observations:
Particles of sugar, salt, Dettol, or potassium permanganate get evenly distributed in water. When we make tea, coffee or lemonade (nimbu paani), particles of one type of matter get into the spaces between particles of the other.
Conclusion:
- There is enough space between particles of matter.
- This space allows other particles to fit in between them.
1.2.2 PARTICLES OF MATTER ARE CONTINUOUSLY MOVING
Observation 1 – Incense Stick:
When an unlit incense stick is placed in a corner:
- You need to go very close to smell it.
When the incense stick is lit:
- The smell spreads throughout the room.
- You can smell it even sitting at a distance.
Observation 2 – Ink and Honey in Water:
When a drop of blue or red ink is added to water:
- It starts spreading slowly.
- Eventually spreads evenly throughout the water.
When honey is added to water:
- It settles at the bottom initially.
- Gradually spreads through the water.
Observation 3 – Effect of Temperature:
When crystals of copper sulphate or potassium permanganate are dropped into glasses of hot water and cold water:
- The colour spreads faster in hot water.
- The colour spreads slowly in cold water.
Conclusions:
- Particles of matter are continuously moving — they possess kinetic energy.
- As the temperature rises, particles move faster.
- With increase in temperature, the kinetic energy of particles increases.
Diffusion:
- The intermixing of particles of two different types of matter on their own is called diffusion.
- On heating, diffusion becomes faster because particles gain more kinetic energy.
1.2.3 PARTICLES OF MATTER ATTRACT EACH OTHER
Human Chain Experiment:
When groups of students form chains with different levels of contact:
- Locked arms (strongest hold) — hardest to break.
- Holding hands (moderate hold) — easier to break.
- Finger tips touching (weakest hold) — easiest to break.
Breaking Different Materials:
When trying to break an iron nail, chalk, and rubber band:
- Iron nail — very difficult to break (strongest force between particles).
- Chalk — breaks easily (moderate force).
- Rubber band — stretches but difficult to break (moderate but flexible force).
Cutting Water Surface:
When trying to cut the surface of water with fingers:
- The surface remains together.
- Water cannot be easily “cut.”
- This is due to force of attraction between water particles.
Conclusion:
- Particles of matter have force acting between them.
- This force keeps the particles together.
- The strength of this force varies from one kind of matter to another.
Questions
Question 1: Which of the following are matter?
- Chair, air, love, smell, hate, almonds, thought, cold, lemon water, smell of perfume.
Solution:
- Matter: Chair, air, almonds, lemon water.
- Not matter: Love, smell (it’s a sensation caused by matter particles), hate, thought, cold (sensation), smell of perfume (sensation, though caused by perfume particles in air).
Question 2: Give reasons for the following observation:
The smell of hot sizzling food reaches you several metres away, but to get the smell from cold food you have to go close.
Solution:
- In hot food, particles have more kinetic energy and move faster.
- They diffuse quickly into the air and reach us rapidly.
- In cold food, particles have less kinetic energy and move slowly.
- They diffuse slowly, so we need to go closer to smell it.
Question 3: A diver is able to cut through water in a swimming pool. Which property of matter does this observation show?
Solution:
- This shows that particles of matter have spaces between them.
- The diver’s body can move through these spaces.
- Water particles are not rigidly fixed, they can move around, allowing the diver to pass through.
Question 4: What are the characteristics of the particles of matter?
Solution:
- Particles of matter are very small (beyond imagination).
- Particles of matter have spaces between them.
- Particles of matter are continuously moving (possess kinetic energy).
- Particles of matter attract each other (have force of attraction).
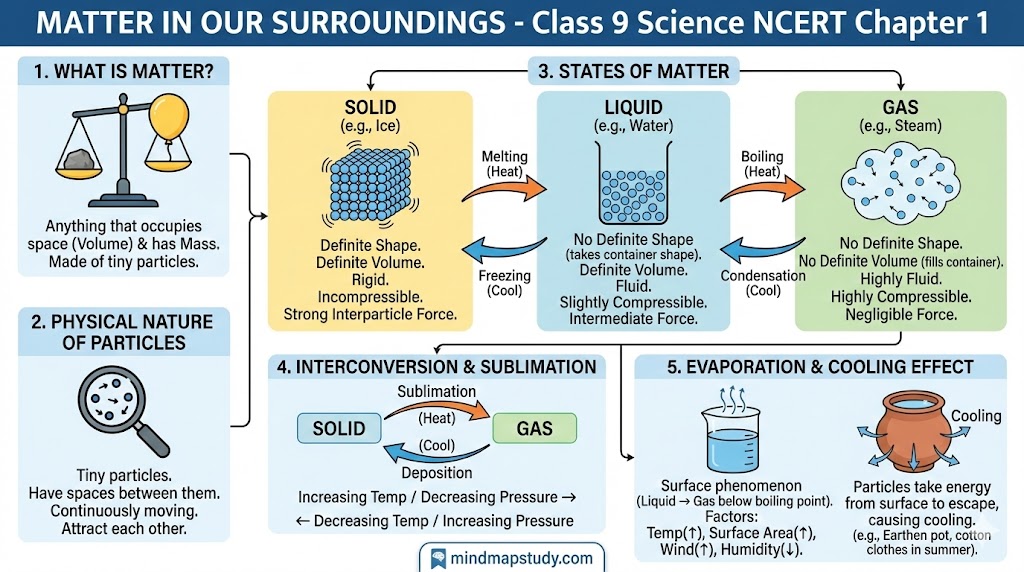
1.3 States of Matter
Matter around us exists in three different states:
- Solid
- Liquid
- Gas
These states arise due to the variation in characteristics of the particles of matter.
1.3.1 THE SOLID STATE
Properties of Solids:
Examples include: pen, book, needle, wooden stick.
- Have a definite shape.
- Have distinct boundaries.
- Have fixed volume.
- Have negligible compressibility.
- Maintain their shape when subjected to outside force.
- May break under force but difficult to change their shape.
- Are rigid (cannot flow).
Special Cases:
(a) Rubber Band:
- Is a solid.
- Changes shape under force.
- Regains the same shape when force is removed.
- If excessive force is applied, it breaks.
(b) Sugar and Salt:
- Are solids.
- The shape of each individual crystal remains fixed.
- When kept in jars, the collection of crystals takes the shape of the container, but each crystal maintains its own shape.
(c) Sponge:
- Is a solid.
- Has minute holes in which air is trapped.
- When pressed, the air is expelled out, making it appear compressible.
- The solid part of the sponge is not compressed.
1.3.2 THE LIQUID STATE
Properties of Liquids:
Examples include: water, cooking oil, milk, juice, cold drink.
- Have no fixed shape.
- Have fixed volume.
- Take up the shape of the container in which they are kept.
- Flow and change shape.
- Are not rigid but are fluid.
Diffusion in Liquids:
- Solids and liquids can diffuse into liquids.
- Gases from the atmosphere also diffuse and dissolve in water.
- These gases, especially oxygen and carbon dioxide, are essential for survival of aquatic animals and plants.
- Aquatic animals breathe under water due to dissolved oxygen in water.
Rate of Diffusion:
- The rate of diffusion of liquids is higher than that of solids.
- This is because in the liquid state, particles move freely and have greater space between each other compared to solid state.
1.3.3 THE GASEOUS STATE
Observation:
- A balloon seller can fill a large number of balloons from a single cylinder of gas.
- This is possible due to the high compressibility of gases.
Compressibility Experiment:
When three syringes are filled with gas (air), water, and chalk pieces respectively, and pistons are pushed:
- Gas (air): Piston easily pushed in — highly compressible.
- Water (liquid): Piston pushed in slightly — slightly compressible.
- Chalk (solid): Piston cannot be pushed in — negligible compressibility.
Properties of Gases:
- Are highly compressible compared to solids and liquids.
- Liquefied petroleum gas (LPG) and oxygen cylinders contain compressed gas.
- Compressed natural gas (CNG) is used as fuel in vehicles.
- Due to high compressibility, large volumes of gas can be compressed into small cylinders for easy transport.
Diffusion in Gases:
- The smell of cooked food reaches us from the kitchen without entering it.
- Particles of aroma mix with air particles and spread rapidly.
- The smell of hot cooked food reaches us in seconds.
- Due to high speed of particles and large space between them, gases diffuse very fast into other gases.
Pressure Exerted by Gases:
- In the gaseous state, particles move about randomly at high speed.
- Particles hit each other and also the walls of the container.
- The pressure exerted by gas is due to this force per unit area on the container walls.
Questions
Question 1: The mass per unit volume of a substance is called density.
textDensity = Mass / Volume
Arrange the following in order of increasing density:
Air, exhaust from chimneys, honey, water, chalk, cotton, iron.
Solution:
Increasing order of density:
Air → Exhaust from chimneys → Cotton → Water → Honey → Chalk → Iron
Question 2:
(a) Tabulate the differences in the characteristics of states of matter.
| Characteristic | Solid | Liquid | Gas |
|---|---|---|---|
| Shape | Definite shape | No definite shape, takes shape of container | No definite shape, fills entire container |
| Volume | Definite volume | Definite volume | No definite volume, expands to fill container |
| Rigidity | Rigid, cannot flow | Not rigid, can flow | Not rigid, can flow |
| Compressibility | Negligible | Very small | High |
| Fluidity | Cannot flow | Can flow | Can flow easily |
| Kinetic Energy | Minimum | Moderate | Maximum |
| Density | High | Moderate | Low |
| Spaces between particles | Very small | Moderate | Very large |
| Force of attraction | Maximum | Moderate | Minimum |
| Arrangement | Highly ordered | Less ordered | Completely random |
(b) Comment upon the following:
- Rigidity: Solids are rigid because particles are tightly packed and cannot move freely. Liquids and gases are not rigid as particles can move.
- Compressibility: Gases are highly compressible because of large spaces between particles. Liquids have very small compressibility. Solids have negligible compressibility.
- Fluidity: Liquids and gases can flow because particles can move past each other. Solids cannot flow as particles are fixed in position.
- Filling a gas container: Gases fill the entire container because particles move randomly at high speed in all directions.
- Shape: Solids have definite shape due to strong forces between particles. Liquids and gases take the shape of their container.
- Kinetic energy: Gases have maximum kinetic energy (particles move fastest), solids have minimum (particles only vibrate), liquids have moderate kinetic energy.
- Density: Solids have highest density (particles closely packed), gases have lowest density (particles far apart), liquids have moderate density.
Question 3: Give reasons:
(a) A gas fills completely the vessel in which it is kept.
Solution:
- Gas particles have very high kinetic energy and move randomly at high speed.
- There are large spaces between gas particles.
- Particles move in all directions and spread throughout the available space.
- Therefore, gas fills the entire vessel.
(b) A gas exerts pressure on the walls of the container.
Solution:
- Gas particles move randomly at high speed.
- They continuously collide with the walls of the container.
- These collisions exert force per unit area on the walls.
- This force per unit area is called pressure.
(c) A wooden table should be called a solid.
Solution:
- A wooden table has a definite shape and definite volume.
- It is rigid and has negligible compressibility.
- It maintains its shape under normal conditions.
- Therefore, it is a solid.
(d) We can easily move our hand in air but to do the same through a solid block of wood we need a karate expert.
Solution:
- In air (gas), particles are far apart with large spaces between them.
- Our hand can easily move through these spaces.
- In a solid block of wood, particles are tightly packed with very small spaces.
- There is strong force of attraction between particles.
- It is extremely difficult to move through solid matter.
- A karate expert uses technique and force to break the wood, not pass through it.
Question 4: Liquids generally have lower density as compared to solids. But you must have observed that ice floats on water. Find out why.
Solution:
- When water freezes to form ice, it expands and occupies more volume.
- The same mass now occupies larger volume.
- Therefore, density of ice (0.92 g/cm³) is less than density of water (1 g/cm³).
- Since ice is less dense than water, it floats on water.
- This is an exceptional property of water.
1.4 Can Matter Change its State?
Water can exist in three states of matter:
- Solid — as ice
- Liquid — as water
- Gas — as water vapour
Key Questions:
- What happens inside the matter during change of state?
- What happens to the particles during the change?
- How does this change take place?
1.4.1 EFFECT OF CHANGE OF TEMPERATURE
Process of Heating Ice:
When ice is heated:
- Ice starts melting at a specific temperature.
- All ice converts into water.
- Water starts boiling at another specific temperature.
- Water vaporises into steam.
Melting (Fusion)
Process:
When temperature of solids increases:
- The kinetic energy of particles increases.
- Particles start vibrating with greater speed.
- Energy supplied by heat overcomes the forces of attraction between particles.
- Particles leave their fixed positions and start moving more freely.
- The solid melts and converts to liquid.
Melting Point:
- The minimum temperature at which a solid melts to become liquid at atmospheric pressure is called its melting point.
- The melting point indicates the strength of force of attraction between particles.
- Melting point of ice is 273.15 K (0°C).
Fusion:
- The process of melting (change of solid state into liquid state) is also known as fusion.
Latent Heat of Fusion
Observation:
- When a solid melts, its temperature remains constant even though heat is being supplied.
Question: Where does the heat energy go?
Answer:
- During melting, temperature does not change after melting point is reached until all ice melts.
- This heat is used to overcome forces of attraction between particles (changing the state).
- This heat energy is absorbed without showing rise in temperature.
- It is considered hidden in the substance.
Latent Heat:
- The word “latent” means “hidden”.
- Latent heat of fusion is the amount of heat energy required to change 1 kg of solid into liquid at atmospheric pressure at its melting point.
Important Point:
- Particles in water at 0°C (273 K) have more energy than particles in ice at the same temperature.
- This extra energy is the latent heat of fusion absorbed by water.
Boiling (Vaporisation)
Process:
When heat energy is supplied to water:
- Particles start moving even faster.
- At a certain temperature, particles have enough energy to break free from forces of attraction.
- The liquid starts changing into gas.
Boiling Point:
- The temperature at which liquid starts boiling at atmospheric pressure is called its boiling point.
- Boiling is a bulk phenomenon — particles from the bulk (whole) of the liquid gain enough energy to change into vapour state.
- Boiling point of water is 373 K (100°C = 273 + 100).
Latent Heat of Vaporisation
Definition:
- Latent heat of vaporisation is the amount of heat energy required to change 1 kg of liquid into gas at atmospheric pressure at its boiling point.
Important Point:
- Particles in steam (water vapour) at 373 K (100°C) have more energy than water at the same temperature.
- This extra energy is the latent heat of vaporisation absorbed by steam.
Temperature Scales
Kelvin Scale:
- Kelvin is the SI unit of temperature.
- 0°C = 273.15 K (For convenience, we take 0°C = 273 K).
Conversion:
- Kelvin to Celsius: Subtract 273 from the given temperature.
- K – 273 = °C
- Celsius to Kelvin: Add 273 to the given temperature.
- °C + 273 = K
Conclusion:
- The state of matter can be changed by changing the temperature.
Sublimation and Deposition
Observation with Camphor:
When camphor is heated:
- It changes directly from solid to gas (camphor vapours).
- On cooling, vapours change directly back to solid.
- It does not become liquid at any stage.
Definitions:
- Sublimation: A change of state directly from solid to gas without changing into liquid state.
- Deposition: The direct change of gas to solid without changing into liquid state.
Examples of substances that sublime:
- Camphor
- Naphthalene (mothballs)
- Ammonium chloride
- Iodine
- Dry ice (solid CO₂)
1.4.2 EFFECT OF CHANGE OF PRESSURE
Question: What happens when we compress a gas in a cylinder? Will particles come closer? Can pressure change the state of matter?
Effect of Pressure:
By applying pressure, particles of matter can be brought close together.
- Applying pressure and reducing temperature can liquefy gases.
Solid Carbon Dioxide (Dry Ice):
- Solid CO₂ is stored under high pressure.
- On decrease of pressure to 1 atmosphere, solid CO₂ converts directly into gaseous state without becoming liquid.
- This is why solid carbon dioxide is called “dry ice”.
Units:
- Atmosphere (atm) is a unit of measuring pressure.
- Unit of pressure: Pascal (Pa)
- 1 atmosphere = 1.01 × 10⁵ Pa
- Atmospheric pressure is the pressure of air in atmosphere.
- Atmospheric pressure at sea level is 1 atmosphere (normal atmospheric pressure).
Conclusion:
- Pressure and temperature determine the state of a substance (solid, liquid, or gas).
Interconversion of States of Matter
Process Diagram:
text Fusion/Melting (heating)
SOLID ←――――――――――――――――――――→ LIQUID
↑ Solidification (cooling) ↑
| |
| Sublimation | Vaporisation/
| (heating) | Boiling (heating)
| |
| Deposition (cooling) ↓ Condensation
└────────────→ GAS ←─────────────┘ (cooling)
Processes:
- Fusion (Melting): Solid → Liquid (heating)
- Solidification (Freezing): Liquid → Solid (cooling)
- Vaporisation (Boiling): Liquid → Gas (heating)
- Condensation: Gas → Liquid (cooling)
- Sublimation: Solid → Gas (heating, without liquid state)
- Deposition: Gas → Solid (cooling, without liquid state)
Questions
Question 1: Convert the following temperature to celsius scale:
- (a) 300 K
- (b) 573 K
Solution:
- (a) 300 – 273 = 27°C
- (b) 573 – 273 = 300°C
Question 2: What is the physical state of water at:
- (a) 250°C
- (b) 100°C
Solution:
- (a) 250°C: Gas (steam) — because boiling point of water is 100°C, and 250°C is much higher.
- (b) 100°C: Liquid and Gas coexist — at boiling point, water is converting to steam, so both states exist together.
Question 3: For any substance, why does the temperature remain constant during the change of state?
Solution:
- During change of state, the heat energy supplied is used to overcome the forces of attraction between particles.
- This energy is called latent heat (hidden heat).
- Since the energy is used to change the state and not to increase temperature, the temperature remains constant.
- The energy is absorbed without showing any rise in temperature.
Question 4: Suggest a method to liquefy atmospheric gases.
Solution:
- Increase the pressure on the gas.
- Decrease the temperature of the gas.
- By applying high pressure and lowering temperature, gas particles come closer together.
- Their kinetic energy decreases.
- The gas liquefies (converts to liquid state).
1.5 Evaporation
Observation:
- Water left uncovered slowly changes into vapour.
- Wet clothes dry up.
Question: Do we always need to heat or change pressure for changing state? Can change of state happen without reaching boiling point?
Answer: Yes, through evaporation.
Understanding Evaporation:
- Particles of matter are always moving and never at rest.
- At any given temperature, particles have different amounts of kinetic energy.
- In liquids, a small fraction of particles at the surface have higher kinetic energy.
- These particles are able to break away from forces of attraction.
- They get converted into vapour.
Evaporation:
- The phenomenon of change of liquid into vapours at any temperature below its boiling point is called evaporation.
1.5.1 FACTORS AFFECTING EVAPORATION
Experiments:
When 5 mL of water is kept:
- In a test tube near a window/under a fan
- In an open china dish near a window/under a fan
- In an open china dish inside a cupboard
Observation: Water evaporates at different rates in different conditions.
Factors That Increase Rate of Evaporation
The rate of evaporation increases with:
(1) Increase of Surface Area:
- Evaporation is a surface phenomenon.
- If surface area increases, rate of evaporation increases.
- Example: While putting clothes for drying, we spread them out to increase surface area.
(2) Increase of Temperature:
- With increase of temperature, more particles get enough kinetic energy to escape into vapour state.
- Example: Water in hot sun evaporates faster than in shade.
(3) Decrease in Humidity:
- Humidity is the amount of water vapour present in air.
- Air cannot hold more than a definite amount of water vapour at a given temperature.
- If humidity is already high, rate of evaporation decreases.
- Example: Clothes take longer to dry on rainy days (high humidity).
(4) Increase in Wind Speed:
- On windy days, clothes dry faster.
- With increase in wind speed, water vapour particles move away with the wind.
- This decreases the amount of water vapour in the surrounding.
- Example: Clothes dry faster when hung outside on a windy day.
1.5.2 HOW DOES EVAPORATION CAUSE COOLING?
Mechanism:
- In an open vessel, liquid keeps evaporating.
- Particles of liquid absorb energy from the surrounding to regain energy lost during evaporation.
- This absorption of energy from surroundings makes the surroundings cold.
Examples:
(1) Acetone on Palm:
- When acetone (nail polish remover) is poured on palm:
- Particles gain energy from palm and evaporate.
- The palm feels cool.
(2) Sprinkling Water:
- After a hot sunny day, people sprinkle water on roof or ground.
- Large latent heat of vaporisation of water is absorbed from the hot surface.
- This helps cool the hot surface.
(3) Why Wear Cotton Clothes in Summer?
During summer:
- We perspire more (body’s cooling mechanism).
- During evaporation, particles absorb heat energy (latent heat of vaporisation) from body surface.
- This leaves the body cool.
- Cotton is a good absorber of water.
- Cotton absorbs sweat and exposes it to atmosphere for easy evaporation.
- Therefore, cotton clothes keep us cool in summer.
(4) Water Droplets on Cold Glass:
When ice-cold water is kept in a tumbler:
- Water droplets appear on outer surface.
- Water vapour present in air comes in contact with cold glass.
- Water vapour loses energy and gets converted to liquid state.
- We see this as water droplets.
- This process is called condensation.
Questions
Question 1: Why does a desert cooler cool better on a hot dry day?
Solution:
- On a hot dry day, humidity is low (less water vapour in air).
- Water from the cooler evaporates faster in low humidity.
- Faster evaporation causes more cooling.
- Therefore, desert cooler works better on hot dry days.
Question 2: How does the water kept in an earthen pot (matka) become cool during summer?
Solution:
- Earthen pots have tiny pores on their surface.
- Water seeps out through these pores.
- This water evaporates from the surface.
- During evaporation, water absorbs latent heat from the pot and remaining water inside.
- This causes the water inside to become cool.
Question 3: Why does our palm feel cold when we put some acetone or petrol or perfume on it?
Solution:
- Acetone, petrol, and perfume are volatile liquids (evaporate quickly).
- When put on palm, they evaporate rapidly.
- During evaporation, they absorb heat energy from the palm.
- This causes the palm to feel cold.
Question 4: Why are we able to sip hot tea or milk faster from a saucer rather than a cup?
Solution:
- In a saucer, the surface area is larger compared to a cup.
- Larger surface area causes faster evaporation.
- Faster evaporation cools the tea/milk quickly.
- Therefore, we can sip it faster from a saucer.
Question 5: What type of clothes should we wear in summer?
Solution:
- We should wear cotton clothes in summer.
- Cotton is a good absorber of water.
- It absorbs sweat from body.
- Sweat evaporates easily from cotton fabric.
- Evaporation causes cooling effect on the body.
- Cotton clothes keep us cool and comfortable.
Measurable Quantities and Their Units
| Quantity | Unit | Symbol |
|---|---|---|
| Temperature | kelvin | K |
| Length | metre | m |
| Mass | kilogram | kg |
| Weight | newton | N |
| Volume | cubic metre | m³ |
| Density | kilogram per cubic metre | kg m⁻³ |
| Pressure | pascal | Pa |
Exercises
Question 1: Convert the following temperatures to the celsius scale:
- (a) 293 K
- (b) 470 K
Solution:
- (a) 293 – 273 = 20°C
- (b) 470 – 273 = 197°C
Question 2: Convert the following temperatures to the kelvin scale:
- (a) 25°C
- (b) 373°C
Solution:
- (a) 25 + 273 = 298 K
- (b) 373 + 273 = 646 K
Question 3: Give reason for the following observations:
(a) Naphthalene balls disappear with time without leaving any solid.
Solution:
- Naphthalene balls undergo sublimation.
- They change directly from solid to gas without becoming liquid.
- The solid gradually converts to gas and mixes with air.
- Therefore, they disappear without leaving any solid residue.
(b) We can get the smell of perfume sitting several metres away.
Solution:
- Perfume contains volatile substances that evaporate easily.
- The vapour particles diffuse into air.
- Due to continuous movement and high speed of gas particles, they spread rapidly.
- The perfume particles mix with air and reach us even at a distance.
- This is due to diffusion of gas particles.
Question 4: Arrange the following substances in increasing order of forces of attraction between the particles:
Water, sugar, oxygen.
Solution:
Increasing order of forces of attraction:
Oxygen < Water < Sugar
Explanation:
- Oxygen is a gas — has minimum force of attraction (particles far apart).
- Water is a liquid — has moderate force of attraction (particles moderately close).
- Sugar is a solid — has maximum force of attraction (particles tightly packed).
Question 5: What is the physical state of water at:
- (a) 25°C
- (b) 0°C
Solution:
- (a) 25°C: Liquid (normal water at room temperature).
- (b) 0°C: Can be solid or liquid or both (freezing/melting point — ice and water coexist).
Question 6: Give two reasons to justify:
(a) Water at room temperature is a liquid.
Solution:
- Water has no fixed shape but has fixed volume — it takes the shape of container.
- Water can flow easily from one place to another.
(b) An iron almirah is a solid at room temperature.
Solution:
- An iron almirah has a definite shape and definite volume.
- It is rigid and has negligible compressibility — it maintains its shape and cannot be compressed.
Question 7: Why is ice at 273 K more effective in cooling than water at the same temperature?
Solution:
- Both ice and water are at the same temperature (273 K or 0°C).
- However, ice will absorb extra heat in the form of latent heat of fusion to melt into water.
- This extra heat (latent heat) is absorbed from the surroundings.
- Water at 273 K does not need to absorb this extra energy.
- Therefore, ice absorbs more heat from surroundings and provides better cooling than water at the same temperature.
Question 8: What produces more severe burns, boiling water or steam?
Solution:
- Steam produces more severe burns than boiling water.
- Both are at the same temperature (100°C or 373 K).
- However, steam contains extra energy in the form of latent heat of vaporisation.
- When steam comes in contact with skin, it condenses to water.
- During condensation, it releases latent heat of vaporisation.
- This extra energy release causes more severe burns.
- Boiling water only transfers its heat, but steam transfers heat + latent heat.
Question 9: Name A, B, C, D, E and F in the following diagram showing change in its state:
text Increase heat and decrease pressure
↓
E
↓
SOLID ←→ LIQUID ←→ GAS
↑ A B C ↑
|___________________|
F
↑
Decrease heat and increase pressure
Solution:
- A: Fusion (Melting)
- B: Vaporisation (Boiling)
- C: Condensation
- D: Solidification (Freezing)
- E: Sublimation
- F: Deposition
Download Free Mind Map from the link below
This mind map contains all important topics of this chapter
Visit our Class 9 Science page for free mind maps of all Chapters